Carbon Isotope With 7 Neutrons
4.8: Isotopes - When the Number of Neutrons Varies
- Page ID
- 47477
- Explicate what isotopes are and how an isotope affects an element'due south atomic mass.
- Determine the number of protons, electrons, and neutrons of an chemical element with a given mass number.
All atoms of the aforementioned element take the aforementioned number of protons, just some may take different numbers of neutrons. For case, all carbon atoms have six protons, and about have half-dozen neutrons besides. Simply some carbon atoms have seven or 8 neutrons instead of the usual 6. Atoms of the same chemical element that differ in their numbers of neutrons are called isotopes. Many isotopes occur naturally. Usually i or two isotopes of an element are the most stable and common. Different isotopes of an element generally accept the aforementioned physical and chemical backdrop because they have the same numbers of protons and electrons.
An Instance: Hydrogen Isotopes
Hydrogen is an example of an chemical element that has isotopes. Three isotopes of hydrogen are modeled in Figure \(\PageIndex{i}\). Most hydrogen atoms accept simply one proton, i electron, and lack a neutron. These atoms are just called hydrogen. Some hydrogen atoms have one neutron likewise. These atoms are the isotope named deuterium. Other hydrogen atoms have two neutrons. These atoms are the isotope named tritium.
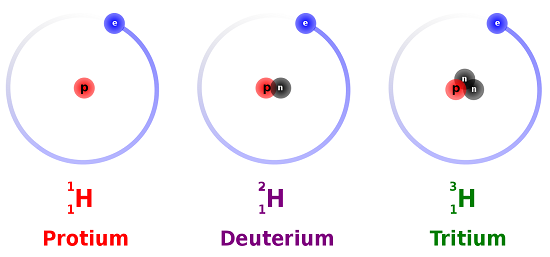
For most elements other than hydrogen, isotopes are named for their mass number. For case, carbon atoms with the usual half-dozen neutrons have a mass number of 12 (6 protons + six neutrons = 12), so they are called carbon-12. Carbon atoms with seven neutrons have an atomic mass of thirteen (6 protons + 7 neutrons = 13). These atoms are the isotope called carbon-13.
- What is the atomic number and the mass number of an isotope of lithium containing 3 neutrons?
- What is the atomic number and the mass number of an isotope of lithium containing 4 neutrons?
Solution
A lithium cantlet contains 3 protons in its nucleus irrespective of the number of neutrons or electrons.
a.
\[ \begin{align}\text{atomic number} = \left( \text{number of protons} \right) &= 3 \nonumber \\ \left( \text{number of neutrons} \correct) &= iii \nonumber\terminate{align} \nonumber \]
\[ \begin{align} \text{mass number} & = \left( \text{number of protons} \right) + \left( \text{number of neutrons} \right) \nonumber\\ \text{mass number} & = iii + iii \nonumber\\ &= 6 \nonumber \end{align}\nonumber \]
b.
\[ \begin{align}\text{diminutive number} = \left( \text{number of protons} \correct) &= three \nonumber\\ \left( \text{number of neutrons} \right) & = 4\nonumber\finish{align}\nonumber \]
\[ \begin{align}\text{mass number} & = \left( \text{number of protons} \correct) + \left( \text{number of neutrons} \right)\nonumber \\ \text{mass number} & = 3 + 4\nonumber \\ &= 7 \nonumber \end{align}\nonumber \]
Notice that because the lithium atom always has 3 protons, the atomic number for lithium is always iii. The mass number, still, is 6 in the isotope with 3 neutrons, and 7 in the isotope with 4 neutrons. In nature, merely certain isotopes be. For instance, lithium exists as an isotope with iii neutrons, and as an isotope with four neutrons, but it doesn't exist as an isotope with 2 neutrons or as an isotope with v neutrons.
Stability of Isotopes
Atoms need a certain ratio of neutrons to protons to take a stable nucleus. Having too many or too few neutrons relative to protons results in an unstable, or radioactive, nucleus that will sooner or later break downwards to a more stable grade. This process is chosen radioactive decay. Many isotopes have radioactive nuclei, and these isotopes are referred to as radioisotopes. When they decay, they release particles that may exist harmful. This is why radioactive isotopes are dangerous and why working with them requires special suits for protection. The isotope of carbon known as carbon-14 is an example of a radioisotope. In contrast, the carbon isotopes chosen carbon-12 and carbon-13 are stable.
This whole discussion of isotopes brings u.s. back to Dalton's Atomic Theory. Co-ordinate to Dalton, atoms of a given element are identical. But if atoms of a given element can have dissimilar numbers of neutrons, then they can have unlike masses equally well! How did Dalton miss this? It turns out that elements found in nature exist as abiding uniform mixtures of their naturally occurring isotopes. In other words, a piece of lithium always contains both types of naturally occurring lithium (the type with three neutrons and the type with 4 neutrons). Moreover, it always contains the 2 in the same relative amounts (or "relative affluence"). In a clamper of lithium, \(93\%\) volition always be lithium with 4 neutrons, while the remaining \(seven\%\) will always be lithium with iii neutrons.
Dalton always experimented with large chunks of an element—chunks that contained all of the naturally occurring isotopes of that element. Every bit a result, when he performed his measurements, he was actually observing the averaged properties of all the dissimilar isotopes in the sample. For most of our purposes in chemical science, we will practise the same thing and deal with the average mass of the atoms. Luckily, aside from having different masses, almost other backdrop of different isotopes are similar.
There are two main ways in which scientists frequently show the mass number of an atom they are interested in. It is important to note that the mass number is non given on the periodic table. These two ways include writing a nuclear symbol or by giving the proper noun of the element with the mass number written.
To write a nuclear symbol, the mass number is placed at the upper left (superscript) of the chemical symbol and the atomic number is placed at the lower left (subscript) of the symbol. The complete nuclear symbol for helium-four is drawn below:

The following nuclear symbols are for a nickel nucleus with 31 neutrons and a uranium nucleus with 146 neutrons.
\[\ce{^{59}_{28}Ni} \nonumber \]
\[ \ce{ ^{238}_{92}U} \nonumber \]
In the nickel nucleus represented in a higher place, the atomic number 28 indicates that the nucleus contains 28 protons, and therefore, it must contain 31 neutrons in order to have a mass number of 59. The uranium nucleus has 92 protons, every bit all uranium nuclei do; and this particular uranium nucleus has 146 neutrons.
Another way of representing isotopes is by calculation a hyphen and the mass number to the chemic name or symbol. Thus the 2 nuclei would be Nickel-59 or Ni-59 and Uranium-238 or U-238, where 59 and 238 are the mass numbers of the two atoms, respectively. Note that the mass numbers (non the number of neutrons) are given to the side of the name.
How many protons, electrons, and neutrons are in an atom of \(^{40}_{19}\ce{Thou}\)?
Solution
\[\text{atomic number} = \left( \text{number of protons} \right) = nineteen \nonumber \]
For all atoms with no charge, the number of electrons is equal to the number of protons.
\[\text{number of electrons} = 19 \nonumber \]
The mass number, xl, is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
\[\text{number of neutrons} = 40 - xix = 21. \nonumber \]
How many protons, electrons, and neutrons are in an atom of zinc-65?
Solution
\[\text{number of protons} = 30 \nonumber \]
For all atoms with no charge, the number of electrons is equal to the number of protons.
\[\text{number of electrons} = thirty \nonumber \]
The mass number, 65, is the sum of the protons and the neutrons.
To find the number of neutrons, subtract the number of protons from the mass number.
\[\text{number of neutrons} = 65 - xxx = 35 \nonumber \]
How many protons, electrons, and neutrons are in each atom?
- \(^{lx}_{27}\ce{Co}\)
- Na-24
- \(^{45}_{20}\ce{Ca}\)
- Sr-90
- Answer a:
- 27 protons, 27 electrons, 33 neutrons
- Answer b:
- xi protons, 11 electrons, 13 neutrons
- Reply c:
- 20 protons, 20 electrons, 25 neutrons
- Reply d:
- 38 protons, 38 electrons, 52 neutrons
Summary
- The number of protons is always the same in atoms of the same element.
- The number of neutrons tin be different, even in atoms of the aforementioned element.
- Atoms of the same element that contain the aforementioned number of protons, but unlike numbers of neutrons, are known as isotopes.
- Isotopes of any given element all contain the aforementioned number of protons, and then they take the same diminutive number (for example, the atomic number of helium is ever 2).
- Isotopes of a given element contain different numbers of neutrons, therefore, different isotopes have different mass numbers.
Carbon Isotope With 7 Neutrons,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/04%3A_Atoms_and_Elements/4.08%3A_Isotopes_-_When_the_Number_of_Neutrons_Varies
Posted by: hobgoodplas1968.blogspot.com


0 Response to "Carbon Isotope With 7 Neutrons"
Post a Comment